Dino Medical – Leading Liposuction Cannula Manufacturer | Years of Specialized R&D
Deciding on a reliable liposuction cannula manufacturer is of utmost importance for surgeons, cosmetic clinics, OEM brands, and medical distributors. Cannulas are Class I or Class II medical devices, depending on the jurisdiction where they are used, based on how they are manufactured; so, any deviation from the specifications on the cannula can affect the way it performs surgically and how safe it is for the patient.
It is essential for the right manufacturer to provide proof of regulatory compliance via documentation that demonstrates that they have validated their manufacturing processes, and documentation that proves that the supplier has the capability to manufacture products with consistent quality across a large lot size.
This article will provide you with guidelines that you can use to evaluate liposuction cannula manufacturers to help you determine which supplier to use when you are ready to purchase your bulk order.
So, let's get started.
Before getting straight to how to choose the right Liposuction Cannula manufacturers, it's essential to first understand the importance of choosing the right one.
Choosing the correct manufacturer will have a direct impact on the success of the surgical procedure, as well as on the long-term performance of the clinic. Cannulas will always be exposed to some mechanical forces. Therefore, if there are problems with the size of the tubing or the mechanical stiffness of the tubing, the result will be increased tissue damage, irregular aspiration patterns, and decreased graft survival rates.
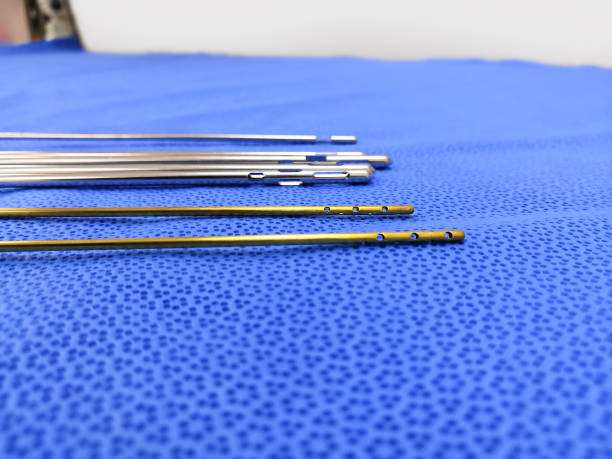
The correct supplier of liposuction cannulas will have documented validation of their manufacturing and polishing processes, and they will maintain very tight tolerances between every cannula that they make, as well as to meet the requirements of international standards such as ISO 13485 and CE MDR or the US FDA. These elements will ensure that the cannula will perform consistently and predictably in a surgical environment.
A high-quality supplier helps you avoid:
1. Inconsistent cannula stiffness, which can reduce surgeon control and increase procedural risk.
2. Rough inner or outer surfaces, leading to higher tissue trauma and postoperative complications.
3. Poorly aligned or imprecise ports, resulting in uneven fat removal or clogging.
4. Unverified materials, which may compromise biocompatibility and sterility.
5. Batch-to-batch variability, which affects surgical outcomes and increases liability for clinics and OEM brands.
By choosing a manufacturer with proven quality systems, reliable traceability, and strong engineering capability, clinics and distributors protect both their patients and their brand reputation.
If you are looking for a manufacturer of liposuction cannula, there are several factors to consider in order to select a reliable manufacturer. The following points will help guide your decision:
One of the first things you should do when selecting a liposuction cannula manufacturer is to check for their certifications. Having the proper certification means that the manufacturer adheres to the Global Medical Standards and produces products that are safe and of high quality, as well as providing traceability and minimising risk for clinics, surgeons, and OEM brands.

When considering certification, the following certifications are recognised in almost every country in the world:
ISO 13485 certification is one of the most important certifications for manufacturers of medical devices. It demonstrates how a manufacturer can control every aspect of production, manage risk to their customers appropriately, and maintain accurate records. For this reason, the majority of Certified Manufacturers that have CE and FDA approvals also hold ISO 13485 certification.
This certification guarantees that the manufacturers have produced their cannulas consistently and safely from the time raw materials were received to the point of final sterilisation.
CE marking indicates that the manufacturer complies with the European safety and clinical requirements. With the introduction of the EU's MDR in 2021, CE marking is now subject to stricter regulations than it was under the previous MDD. In order to receive a valid CE marking, a manufacturer must pass inspections by a recognised "Notified Body", such as TÜV SÜD, BSI, or SGS.
The CE marking ensures that the cannulas manufactured by the manufacturer comply with all the requirements for legal commercialisation throughout Europe.
In the United States, the majority of cannulas are classified as Class II Devices that require FDA Registration and 510(k) Clearances. In addition, the requirement for a manufacturer to follow 21 CFR Part 820 (FDA Quality System Regulations), requires that the manufacturer maintain comprehensive documentation of each step of the production process in order to support their FDA Approval.
The FDA Approval signifies that the cannulas produced by the manufacturer are consistent with the required standards for U.S. safety and efficacy.
ISO 9001 is a quality certification for all types of industries. Even though it does not apply specifically to medical devices, it allows you to see that a manufacturer is managing their operations in a uniform manner and monitoring their processes for continuous improvement.
Many manufacturers hold both ISO 9001 and ISO 13485 together in order to help strengthen their quality management systems.
Certificate validity should always be checked through official registries:
Certificate | How to Verify |
ISO 13485 / ISO 9001 | Verify on the issuing auditor’s website (SGS, TÜV Rheinland, BSI, Intertek). |
CE MDR | Check the notified body’s number on NANDO (EU Commission database). |
FDA 510(k) | Search on FDA 510(k) Premarket Notification Database. |
FDA Registration | Check the FDA Establishment Registration & Device Listing databa |
Any legitimate manufacturer will share certificate numbers, issue dates, and auditor details without hesitation.
When searching for a manufacturer of liposuction cannulas, the materials and process capabilities of the company should top your list of priorities. The composition of materials must be correct, with sufficient mechanical characteristics, and a smooth surface over the entire length of the cannula; otherwise, manufacturers cannot ensure that the product will be safe, and/or of good quality, and that it won't be subject to mechanical failure during use.
Reputable manufacturers typically use:
1. 316L Stainless Steel: High corrosion resistance, biocompatible, and strong. Essential for the cannula tube.
2. Titanium Alloy (Ti-6Al-4V): Lightweight, fatigue-resistant, non-magnetic, and highly durable.
3. PEEK (Polyether Ether Ketone): Used for handles or connectors due to high chemical and thermal resistance, suitable for sterilization.
Reliable manufacturers maintain strict tolerances to ensure precision:
1. Tensile Strength: Stainless steel tubing typically 485–620 MPa.
2. Surface Roughness (Ra): Polished cannulas ≤0.4 µm to reduce tissue trauma.
3. Grinding & Dimensional Tolerances: OD ±0.02–0.04 mm, ID ±0.03–0.05 mm, concentricity <0.03 mm.
1. In-House CNC: Ensures consistent dimensional accuracy, controlled polishing quality, faster custom modifications, and complete traceability.
2. Subcontracting: Can lead to batch variability, inconsistent quality, and less transparency in materials and processes.
For surgeons, clinics, and OEM brands, manufacturers with proven in-house material control and machining capability are always preferred. This ensures safe, precise, and durable liposuction cannulas for clinical and surgical use.
A reliable liposuction cannula manufacturer will have a comprehensive, documented quality control (QC) program in place that ensures each cannula manufactured meets all safety and performance standards to protect the patient and maintain the manufacturer's brand image. Manufacturers with a strong reputation in the industry will have defined checkpoints throughout the entire manufacturing process for consistent QC.

1. Incoming Raw Material Testing
1. Verify chemical composition using XRF or spectrometers.
2. Conduct tensile or hardness testing according to ASTM standards.
3. Cross-check Certificates of Analysis to confirm material quality.
2. In-Process QC
1. Use CMM (Coordinate Measuring Machine) to measure OD, ID, and length.
2. Inspect tip geometry, including bevel angle and port size consistency.
3. Check straightness; maximum deviation is usually <0.3 mm.
3. Final Inspection
1. 100% visual check under magnification.
2. Verify surface finish for smoothness.
3. Test connector-thread fit and packaging seal integrity (ASTM F88/F1929).
4. Perform sterility validation for EO-sterilized items.
5. Maintain batch records for complete traceability.
A robust QC system ensures that each batch of cannulas performs consistently in surgical procedures.
When companies are looking for a manufacturer, one of the biggest factors they use to decide which one to work with is whether or not the manufacturer has the ability to customize cannulas. A reliable manufacturer has the ability to provide both OEM (original equipment manufacturer) and ODM (original design manufacturer) services to meet the clinical and marketing needs of the distributor, clinic, or OEM brand.

Typical Customization Options
1. Custom lumen shapes: Mercedes, V-port, spiral, multi-hole, or dispersion.
2. Wall thickness adjustments for controlled stiffness.
3. Shaft length variations to match surgical preferences.
4. Handle colors for easy size coding.
5. Branding on handles, connectors, or packaging.
6. Private-label sterile packaging for clinic or distributor branding.
Manufacturers with strong Liposuction Cannula OEM capabilities can produce high-quality, tailored cannulas without compromising safety or precision, giving clinics and distributors a competitive advantage.
This table reflects realistic industry numbers (verified across leading surgical manufacturers):
Custom Option | Typical MOQ | Tooling Cost Range | Lead Time |
New Tip Geometry | 300–500 pcs | USD 800–1500 | 20–30 days |
Custom Handle Color | 200 pcs | None | 7–12 days |
Private Label Packaging | 500 sets | USD 200–500 | 12–20 days |
OEM Branding | 300 pcs | USD 100–300 | 10–14 days |
A thorough factory audit of the cannula manufacturer is essential before placing a bulk order to ensure the manufacturer can routinely produce high-quality cannulas for liposuction procedures.

This factory audit checklist is usually used by both the procurement team and the auditor:
1. Cleanroom: ISO Class 7–8 for final assembly/packaging.
2. Sterilization documentation: EO cycle validation (ISO 11135) or Gamma validation (ISO 11137).
3. Equipment list: CNC machines, polishing equipment, ultrasonic cleaning.
4. Calibration records: Micrometers, CMM, hardness testers, torque gauges.
5. Traceability system: Lot codes, material heat numbers.
6. Corrective Action Reports (CAR): For handling defects.
7. Packaging validation tests: Seal strength, dye penetration, aging tests.
A manufacturer that meets these criteria demonstrates the capability to maintain consistent quality, safety, and compliance across all production batches.
Several variables typically determine the pricing of liposuction cannula products: design, finish, and packaging. Understanding how these elements relate to the overall pricing structure can help you compare manufacturers effectively.

1. Raw material cost: Based on 316L tubing market price.
2. Machining cost: CNC time, tool wear, polishing steps.
3. Tooling cost: Only for new lumen or handle designs.
4. Sterilization cost: EO or gamma sterilization fee per batch.
5. Freight and duties: Air freight is most common for sterile items.
6. Quality risk cost: Returns, failed batches, or regulatory non-compliance.
1. 30% deposit + 70% before shipment: Standard in Asia and Europe.
2. Letter of Credit (L/C): Used for high-value orders.
3. Net 30/60 terms: Only for long-term customers.
Always calculate the total landed cost, including shipping, duties, and sterilization, when comparing manufacturers.
Some manufacturers will continue to support your business after the cannulas are delivered, with logistics assistance and services to protect your investment and assist with the ongoing operations of your business.

1. Heat-sealed medical-grade pouches (per ISO 11607).
2. Seal strength testing (ASTM F88).
3. Accelerated aging test for expiry validation.
4. EO residual testing for sterilized products.
Reliable manufacturers provide:
1. Replacement for defective batches.
2. Spare handle kits (PEEK or polymer handles).
3. Lot-number-based tracking assistance.
4. Technical drawings for OEM orders.
Manufacturers with strong logistics and after-sales support minimize downtime, ensure patient safety, and provide confidence in long-term partnerships.
In terms of surgical product quality, China is considered to be the premier manufacturer of surgical instruments due to the availability of high-quality products and low cost of production. Partnering with a reputable Chinese manufacturer, such as Dino Medical, to manufacture your product can give you the most competitive pricing, while providing you with a high level of compliance with current quality standards as well as high levels of precision and automation in the manufacturing process.
1. Proven Expertise & High Production Capacity: With over 15 years of experience, Dino Medical produces millions of cannulas annually, ensuring consistent supply and fast delivery.
2. Full OEM/ODM Services: From custom designs and CNC machining to polishing, EO sterilization, and private-label packaging, Dino Medical offers end-to-end solutions that simplify procurement and guarantee quality.
3. Strong Regulatory Compliance: Their products meet international standards, giving buyers confidence in sterility, material safety, and overall reliability.
4. Flexible Customization Options: Choose from different tip styles, shaft diameters, handle types, or private-label packaging. Dino Medical's flexibility supports clinic branding and OEM needs.
5. Competitive & Scalable Manufacturing: Access to mature stainless tubing supply chains and CNC clusters allows for cost-effective production without compromising precision.
By sourcing from China, especially from a trusted liposuction cannula manufacturer like Dino Medical, buyers gain high-quality, compliant, customizable, and cost-effective cannulas.
Q1. What certifications should a reliable lipo cannula manufacturer have?
Reliable liposuction cannula manufacturers should hold ISO 13485, CE MDR certification, and FDA registration for U.S. distribution. ISO 13485 ensures medical-grade process control, while CE and FDA compliance validate sterility, biocompatibility, documentation, and traceability according to international regulations.
Q2. What is the typical MOQ for a custom lipo cannula OEM?
Most liposuction cannula manufacturers offer OEM customization starting from 200–500 units, depending on tip geometry, branding, or packaging. More complex modifications, such as new lumen design,s may require higher MOQs due to tooling. Lead times usually range between 15 and 30 days for custom batches.
Q3. How long does a factory audit for cannula manufacturers take?
A factory audit for liposuction cannula manufacturers generally requires 4–8 hours, depending on the facility size. Auditors review production flow, CNC capability, sterility documentation, calibration records, and CE/FDA compliance evidence. Large facilities with in-house sterilization may require a full day.
Choosing reliable liposuction cannula manufacturers requires evaluating technical capability, regulatory compliance, QC systems, and customization flexibility. Accurate certificate verification, material traceability, machining precision, and validated sterility processes are essential for clinical safety.
A structured audit and clear expectations for tooling, lead time, and total landed cost help ensure long-term reliability. With strong machining infrastructure and OEM expertise, China remains a leading region for sourcing high-quality cannulas.
Copyright © 2025 Hangzhou Dino Medical Instruments Co., Ltd. | All Rights Reserved
We are here to help you! If you close the chatbox, you will automatically receive a responsefrom us via email. Please be sure to leave yourcontact details so that we can better assist